Back
(128) No, No Nootropics! A Case of Tianeptine and Phenibut Withdrawal/Use Disorder
Saturday, April 26, 2025
9:45 AM – 1:15 PM
Location: Aurora Ballroom Pre-Function, Level 2


Kevin Wang, MD
Resident Physician
University of North Carolina - Chapel Hill, North Carolina
Helen Claire (preferred) West, MD
Associate Professor of Medicine
University of North Carolina at Chapel Hill School of Medicine, North Carolina
Presenter(s)
Non-presenting author(s)
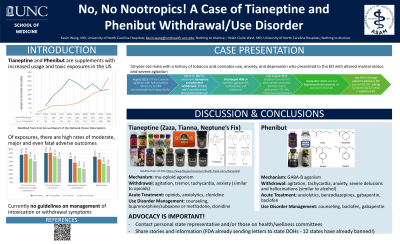
Background & Introduction: Tianeptine – an atypical antidepressant with mu-opioid agonism – and phenibut – a GABA-B agonist initially developed for anxiolysis – are both federally unregulated supplements marketed in the US as nootropics purported to boost mood, promote relaxation, and enhance concentration (1,2). While they may be sold separately, they are often mixed together to enhance their desired effects (2,3).
Both substances possess traits that contribute to use disorder development: accessibility, euphoric effects, bioavailability, short half-lives, and development of tolerance (1,2). Concerningly, their rise in use coincides with increases in reported cases of intoxication and withdrawal in the US. Between 2014 and 2023, there were 2,325 phenibut and 1,392 tianeptine exposures reported to Poison Control centers (4), a sharp rise in use especially for tianeptine, whose case total from 2000-2013 was 11 (3).
Due to these substances’ relative novelty, no consensus exists on the acute management of intoxication or withdrawal. Furthermore, only few case reports exist addressing long-term management of use disorder. This case report aims to highlight a severe case of concurrent tianeptine and phenibut withdrawal, as well as detail the long-term medication management of these rising substance use disorders.
Case Description: A 33-year-old male with a history of tobacco and cannabis use, anxiety, and depression presented to the ED with altered mental status and severe agitation after recent cessation of Zaza Silver – a combination preparation of tianeptine and phenibut. He began use in 2021 after a local smoke shop recommended it for anxiolysis and focus. His use escalated to over 30 capsules per day, with multiple failed attempts to reduce or stop use entirely. Attempts to cut down resulted in insomnia, anxiety, headaches, hypertension, auditory hallucinations, and paranoia, prompting seven prior ED visits in the past 1.5 years for management
During these past ED visits, he was managed with supportive care and discharged with gabapentin, baclofen, and clonidine before being lost to follow-up. However, during this most recent presentation, the severity of his withdrawal warranted ICU stay for high dose benzodiazepines, antipsychotics, and continuous sedation.
He was ultimately discharged on baclofen, gabapentin, hydroxyzine, melatonin and a scheduled visit with a primary care substance use provider. Given the mu-opioid agonism of tianeptine and GABA-B agonism of phenibut, he was treated with buprenorphine/naloxone 8mg/2mg three times daily, along with clonidine, gabapentin, baclofen, and trazodone. He connected with a counselor and support group, and maintained close follow-up with his primary care and addiction team.
Despite ongoing cravings and intermittent Zaza use, he achieved abstinence from tianeptine and phenibut after 3 months of continued follow-up and treatment. He has maintained his medication and follow-up plan for 12 months, achieving his initial goal of family reconciliation.
With the encouragement of his counselors, medical team, and family, he advocated locally for the banning of Zaza sales within his state. By directly speaking with his local representative, he was able to propose a simple amendment to add tianeptine to the Schedule II substance list. He petitioned his providers and contacts to raise awareness to their local representatives, and this bill was unanimously passed and signed into law July 2024.
Conclusion & Discussion: With increased incidence of phenibut and tianeptine withdrawal in the US, it is important for providers to recognize, differentiate, and develop treatment protocols for acute withdrawal. By understanding each substance’s pharmacology, providers can begin to differentiate the opioid-like withdrawal patterns of tianeptine from the alcohol-like GABA-ergic withdrawal of phenibut (2,5). Despite similarities in their withdrawal patterns – agitation, tremor, tachycardia, anxiety/irritability - phenibut withdrawal can uniquely involve severe delusions and hallucinations (1,2,4,5).
Acutely, supportive strategies tailored to these withdrawal patterns can be employed. Some case reports demonstrate that benzodiazepines, clonidine, and opioid agonists such as buprenorphine or methadone are efficacious for tianeptine withdrawal (2). Similarly, case reports of phenibut withdrawal show success with benzodiazepine tapers (1) or baclofen due to its shared GABA-B agonism with phenibut (1,5).
Long-term management of tianeptine and phenibut use disorder in this case was similarly shaped by each nootropic's pharmacology. The partial opioid agonism of buprenorphine/suboxone and clonidine were used to manage the opioid-like cravings of tianeptine (2), and the GABAergic effects of phenibut were addressed with gabapentin and baclofen tapers (1). Combined with substance use counseling, longitudinal primary care, and social support, hopefully similar patients with tianeptine and/or phenibut use disorder can achieve remission.
References: (1) Gurley BJ, Koturbash I. Phenibut: A drug with one too many “buts”. Basic Clin Pharmacol Toxicol. 2024; 135(4): 409-416. doi:10.1111/bcpt.14075
(2) Edinoff, A.N., Sall, S., Beckman, S.P. et al. Tianeptine, an Antidepressant with Opioid Agonist Effects: Pharmacology and Abuse Potential, a Narrative Review. Pain Ther 12, 1121–1134 (2023). https://doi.org/10.1007/s40122-023-00539-5
(3) El Zahran T, Schier J, Glidden E, et al. Characteristics of Tianeptine Exposures Reported to the National Poison Data System — United States, 2000–2017. MMWR Morb Mortal Wkly Rep 2018;67:815–818. DOI: https://dx.doi.org/10.15585/mmwr.mm6730a2.
(4) David D. Gummin, James B. Mowry, Michael C. Beuhler, Daniel A. Spyker, Laura J. Rivers, Ryan Feldman, Kaitlyn Brown, Nathaniel P.T. Pham, Alvin C. Bronstein & Carol DesLauriers (17 Dec 2024): 2023 Annual Report of the National Poison Data System® (NPDS) from America’s Poison Centers®: 41st Annual Report, Clinical Toxicology, DOI: 10.1080/15563650.2024.2412423
(5) Stewart C, Simonsen H, Satyasi SK, Ashraf N, Sukpraprut-Braaten S. A Systematic Review of Phenibut Withdrawals. Cureus. 2024 Sep 6;16(9):e68775. doi: 10.7759/cureus.68775. PMID: 39376891; PMCID: PMC11456982.
Both substances possess traits that contribute to use disorder development: accessibility, euphoric effects, bioavailability, short half-lives, and development of tolerance (1,2). Concerningly, their rise in use coincides with increases in reported cases of intoxication and withdrawal in the US. Between 2014 and 2023, there were 2,325 phenibut and 1,392 tianeptine exposures reported to Poison Control centers (4), a sharp rise in use especially for tianeptine, whose case total from 2000-2013 was 11 (3).
Due to these substances’ relative novelty, no consensus exists on the acute management of intoxication or withdrawal. Furthermore, only few case reports exist addressing long-term management of use disorder. This case report aims to highlight a severe case of concurrent tianeptine and phenibut withdrawal, as well as detail the long-term medication management of these rising substance use disorders.
Case Description: A 33-year-old male with a history of tobacco and cannabis use, anxiety, and depression presented to the ED with altered mental status and severe agitation after recent cessation of Zaza Silver – a combination preparation of tianeptine and phenibut. He began use in 2021 after a local smoke shop recommended it for anxiolysis and focus. His use escalated to over 30 capsules per day, with multiple failed attempts to reduce or stop use entirely. Attempts to cut down resulted in insomnia, anxiety, headaches, hypertension, auditory hallucinations, and paranoia, prompting seven prior ED visits in the past 1.5 years for management
During these past ED visits, he was managed with supportive care and discharged with gabapentin, baclofen, and clonidine before being lost to follow-up. However, during this most recent presentation, the severity of his withdrawal warranted ICU stay for high dose benzodiazepines, antipsychotics, and continuous sedation.
He was ultimately discharged on baclofen, gabapentin, hydroxyzine, melatonin and a scheduled visit with a primary care substance use provider. Given the mu-opioid agonism of tianeptine and GABA-B agonism of phenibut, he was treated with buprenorphine/naloxone 8mg/2mg three times daily, along with clonidine, gabapentin, baclofen, and trazodone. He connected with a counselor and support group, and maintained close follow-up with his primary care and addiction team.
Despite ongoing cravings and intermittent Zaza use, he achieved abstinence from tianeptine and phenibut after 3 months of continued follow-up and treatment. He has maintained his medication and follow-up plan for 12 months, achieving his initial goal of family reconciliation.
With the encouragement of his counselors, medical team, and family, he advocated locally for the banning of Zaza sales within his state. By directly speaking with his local representative, he was able to propose a simple amendment to add tianeptine to the Schedule II substance list. He petitioned his providers and contacts to raise awareness to their local representatives, and this bill was unanimously passed and signed into law July 2024.
Conclusion & Discussion: With increased incidence of phenibut and tianeptine withdrawal in the US, it is important for providers to recognize, differentiate, and develop treatment protocols for acute withdrawal. By understanding each substance’s pharmacology, providers can begin to differentiate the opioid-like withdrawal patterns of tianeptine from the alcohol-like GABA-ergic withdrawal of phenibut (2,5). Despite similarities in their withdrawal patterns – agitation, tremor, tachycardia, anxiety/irritability - phenibut withdrawal can uniquely involve severe delusions and hallucinations (1,2,4,5).
Acutely, supportive strategies tailored to these withdrawal patterns can be employed. Some case reports demonstrate that benzodiazepines, clonidine, and opioid agonists such as buprenorphine or methadone are efficacious for tianeptine withdrawal (2). Similarly, case reports of phenibut withdrawal show success with benzodiazepine tapers (1) or baclofen due to its shared GABA-B agonism with phenibut (1,5).
Long-term management of tianeptine and phenibut use disorder in this case was similarly shaped by each nootropic's pharmacology. The partial opioid agonism of buprenorphine/suboxone and clonidine were used to manage the opioid-like cravings of tianeptine (2), and the GABAergic effects of phenibut were addressed with gabapentin and baclofen tapers (1). Combined with substance use counseling, longitudinal primary care, and social support, hopefully similar patients with tianeptine and/or phenibut use disorder can achieve remission.
References: (1) Gurley BJ, Koturbash I. Phenibut: A drug with one too many “buts”. Basic Clin Pharmacol Toxicol. 2024; 135(4): 409-416. doi:10.1111/bcpt.14075
(2) Edinoff, A.N., Sall, S., Beckman, S.P. et al. Tianeptine, an Antidepressant with Opioid Agonist Effects: Pharmacology and Abuse Potential, a Narrative Review. Pain Ther 12, 1121–1134 (2023). https://doi.org/10.1007/s40122-023-00539-5
(3) El Zahran T, Schier J, Glidden E, et al. Characteristics of Tianeptine Exposures Reported to the National Poison Data System — United States, 2000–2017. MMWR Morb Mortal Wkly Rep 2018;67:815–818. DOI: https://dx.doi.org/10.15585/mmwr.mm6730a2.
(4) David D. Gummin, James B. Mowry, Michael C. Beuhler, Daniel A. Spyker, Laura J. Rivers, Ryan Feldman, Kaitlyn Brown, Nathaniel P.T. Pham, Alvin C. Bronstein & Carol DesLauriers (17 Dec 2024): 2023 Annual Report of the National Poison Data System® (NPDS) from America’s Poison Centers®: 41st Annual Report, Clinical Toxicology, DOI: 10.1080/15563650.2024.2412423
(5) Stewart C, Simonsen H, Satyasi SK, Ashraf N, Sukpraprut-Braaten S. A Systematic Review of Phenibut Withdrawals. Cureus. 2024 Sep 6;16(9):e68775. doi: 10.7759/cureus.68775. PMID: 39376891; PMCID: PMC11456982.
Learning Objectives:
- identify and differentiate the unique features of tianeptine and phenibut withdrawal.
- understand how nootropic pharmacology can guide treatment plans for tianeptine and phenibut withdrawal and use disorder .
- devise advocacy plans to better regulate the sale and distribution of nootropic drugs.
